SPM 2010 Chemistry Tips Analysis
There is document - SPM 2010 Chemistry Tips Analysis available here for reading and downloading. Use the download button below or simple online reader.
The file extension - PDF and ranks to the Study Guides, Notes, & Quizzes category.
Tags
Related
Comments
Log in to leave a message!
Description
Download SPM 2010 Chemistry Tips Analysis
Transcripts
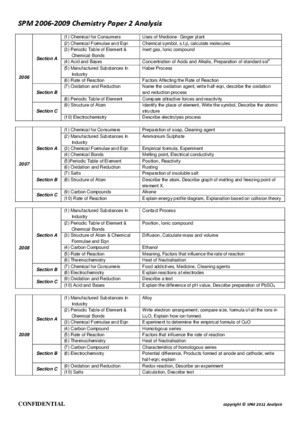
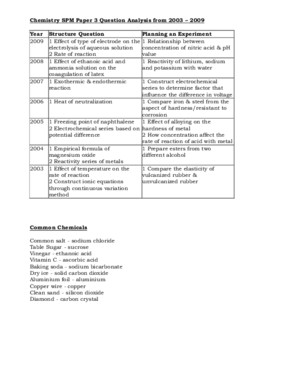
SPM 2010 CHEMSITRY PAPER 2 TIPS ANALYSIS #1 SPM CHEMBLOG [http://spmchemblogspotcom/2010/11/spm-2010-focushtml] SECTION A 1 Structure of atom /naphthalene2 Electrolysis / Chemical cell3 Salt analysis / Titration4 Redox : U-tube5 Manufactured Substances in Industries + Consumer – Contact process, soap Detergent / food addictives6 Carbon compound (flow chart) SECTION B SECTION C - Heat of Combustion – graph / energy level diagram- Empirical formula MgOPeriodic Table / Chemical bond- Acid Base + Preparation of soluble salt- Rate of reaction – comparing rate of reaction #2 Andrew Choo [http://wwwandrewchooedumy/] SECTION A 1 Empirical formula of CuO / MgO2 Periodic Table of Element3 Electrolysis + Electrochemistry4 Carbon compound > schematic diagram5 Contact Haber6 U tube + Displacement of halide by halogen Extra Tips - Salt analysis- Soap / Detergent + Modern medicine SECTION B 7 Rate of Reaction > Concentration / TSA/V8 Bonding > Ionic / Covalent SECTION C 9 Acidity / Strength of acid / Preparation of salt10 HOC / Endo + exo #3 Berry Berry [http://berryberryeasycom/2010/09/spm-chemistry-2010-tips-and-predictions-for-papers-45411-45412-and-45413/] Form 4 Syllabus Chapter 2 *The Structure of the Atom Chapter 3 Chemical Formulae and Equations Chapter 4 Periodic Table of Elements Chapter 5 *Chemical Bonds Chapter 6 Electrochemistry Chapter 9 Manufactured substances in Industry Form 5 Syllabus Chapter 1 *Rate of reaction Chapter 2 Carbon compounds Chapter 3 Oxidation and Reduction Chapter 4 Thermochemistry Chapter 5 *Chemicals for consumers Not Important Chapter 7 Acid and Bases Chapter 8 Salts SPM 2010 CHEMSITRY PAPER 2 TIPS ANALYSIS #4 Mr Sai Mun [http://mrsaimunblogspotcom/2010/11/chemistry-spotted-questions-spm-2010html] SECTION A Q1Q2Q3(i)Q3(ii)Q4Q5Q6(i)Q6(ii)Glass, Haber Process, Prepare Soap and DetergentAtoms, Isotopes, Electrons, Group 1 + WaterElectrolysis Concentration, Voltaic Cell with Half EquationsNeutralisation, calculation + pH with different solventInsoluble Salt, Zinc Carbonate (Heating and Producing), apparatusRedox – Displacement of halides, Testing of Br2,I2,Cl2, U-tubeCarbon Compound-esterification, dehydration, … More updates soon??? SECTION B Q7(i)Q7(ii)Q8Build ECS, Concentration of SolutionPercentage of Composition, Experiment Empirical Formula, Compare and CalculateHeat of Combustion, lab Experiment, Calculations SECTION C Q9Q10Dilution, Lab experiment, calculationsRate of Reaction, Sodium thioshulpate with HCL or H2O2, Collision Theory, Compare #5 CH’NG TUITION [http://chngtuitionblogspotcom/2009/11/2009-spm-chemistry-tipshtml] SECTION A 1 F4 (C9) Contact process and Haber process (equation and condition)2 F4 (C6) Compare electrolytic cell and voltaic cell3 F4 (C7) Prepare standard solution and dilution experiment and calculationTitration experiment and calculation4 F5 (C2) Chemical reaction from alkene to ester (equation and condition)5 F5 (C3) U-tube (flow of electron, terminal of electrodes, function of H 2 SO 4 , observations and equations)(Oxidising agent: KMnO 4 , K 2 Cr 2 O 7 , Br 2 , Cl 2 )(Reducing agent: FeSO 4 , KI)6 F5(C25)Latex (Structure of monomer and polymer and explain its elasticity and vulcanisation)Explain how latex coagulate when exposed to air and how to prevent the coagulationSoap (preparation equation, cleansing process and effectiveness in hard water and soft water) SECTION B 7 F4(C45)Compare Group 1 and Group 17 elementsPhysical properties of Group 17 or reactivity of Group 17 elements towards IronCompare physical properties of ionic compounds and covalent compounds(Electrical conductivity – experiment)8 F5 (C1) Explain the factors that affect the rate of reaction(size, temp concentration, catalyst) by using collisiontheorySketch energy level diagram, equations and calculations SECTION C 9 F4 (C8) Heating of carbonate saltExperiment: Prepare soluble salt / insoluble salt10 F5 (C3) Compare and explain exothermic reaction and endothermic reactionExperiment: Heat of neutralisation between weak acid and strong alkali SPM 2010 CHEMSITRY PAPER 2 TIPS ANALYSIS Chemistry [PAPER 2] SECTION A 1 Empirical formula (precaution, draw apparatus, observation, calculation) 2 Periodic table 3 Carbon compound (alkane, alkane, alcohol) 4 Salt 5 Redox and electrolysis (define term) 6 Soap and detergent (how to make soap, adv, disadvantage) 7 Contact Haber process 8 Thermochemistry SECTION B 1 How to make soluble / insoluble salt (Identity anion / cation) 2 Rate of reaction (catalyst, surface area, concentration) 3 Bond (ionic , covalent) 4 Combination of (alloy ,glass, polymer) or Rubber (polymer vulcanisation)
Recommended